Gilero offers flexible contract manufacturing services to meet changing production demands, reduce lead times, and support growth at scale. We support everything from small batch builds to full-scale commercial production for drug delivery systems, medical devices, and diagnostics.
Accelerating the path to market for next-generation medical Devices.
Located about 30 miles west of Durham, the Pittsboro facility specializes in assembly, testing, and packaging for medical devices, supporting design validation, clinical builds, and new product introduction.

This site can handle pharmaceuticals and manufacture combination products.

Features on-site ISO 7 and 8 cleanrooms for device assembly and packaging.

FDA-registered, ISO 13485 certified, and cGMP-compliant.

High-volume production meets cleanroom precision for sterile and non-sterile medical devices.
Gilero’s 35,000-square-foot Tijuana facility supports scalable manufacturing for a wide range of medical devices. The facility is FDA-registered, ISO 13485 certified, and cGMP-compliant, maintaining consistent quality across high-volume production.

The facility accommodates large production volumes across a variety of medical devices, supporting long-term production needs with built-in flexibility.

The Tijuana site has two ISO Class 8 cleanrooms totaling 14,000 square feet provide controlled environments for sterile and non-sterile device assembly and packaging.

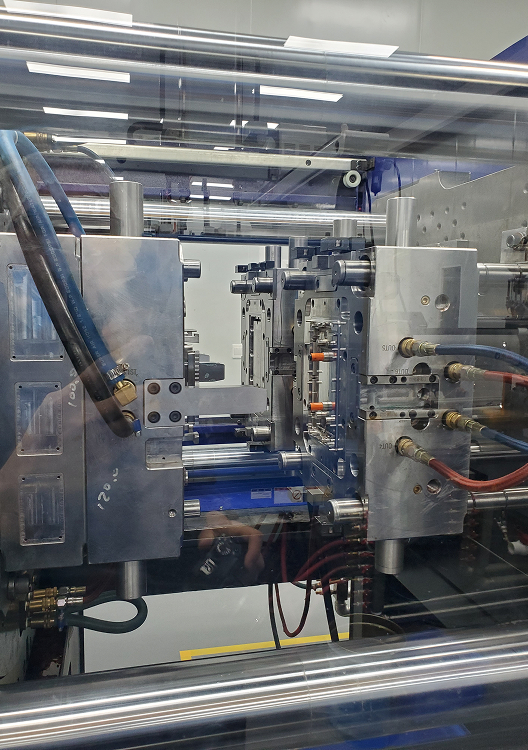
US-based injection molding for medical and pharmaceutical products.
Opened in 2025, our 60,000-square-foot Greensboro facility expands U.S. manufacturing capacity to meet growing demand for pharmaceutical and combination products. The site plays a key role in producing components, sub-assemblies, and embedded desiccants for moisture-sensitive medical devices.

The facility offers fully-electric thermoplastic injection molding with two-shot capabilities and the ability to use a wide range of materials.

The Greensboro site has state-of-the-art cleanroom and white room spaces with automated environmental monitoring.

Global infrastructure for advanced injection molding and assembly.
The Sanner Group operates more than a dozen facilities across North America, Europe, and Asia, supporting device manufacturing at every stage of development.

All sites operate under cGMP, FDA, EMA, and ISO 13485 standards, ensuring consistent regulatory compliance and product quality.

Additionally, Sanner manufactures moisture management solutions in the form of drop-in, fit-in, and built-in desiccants.