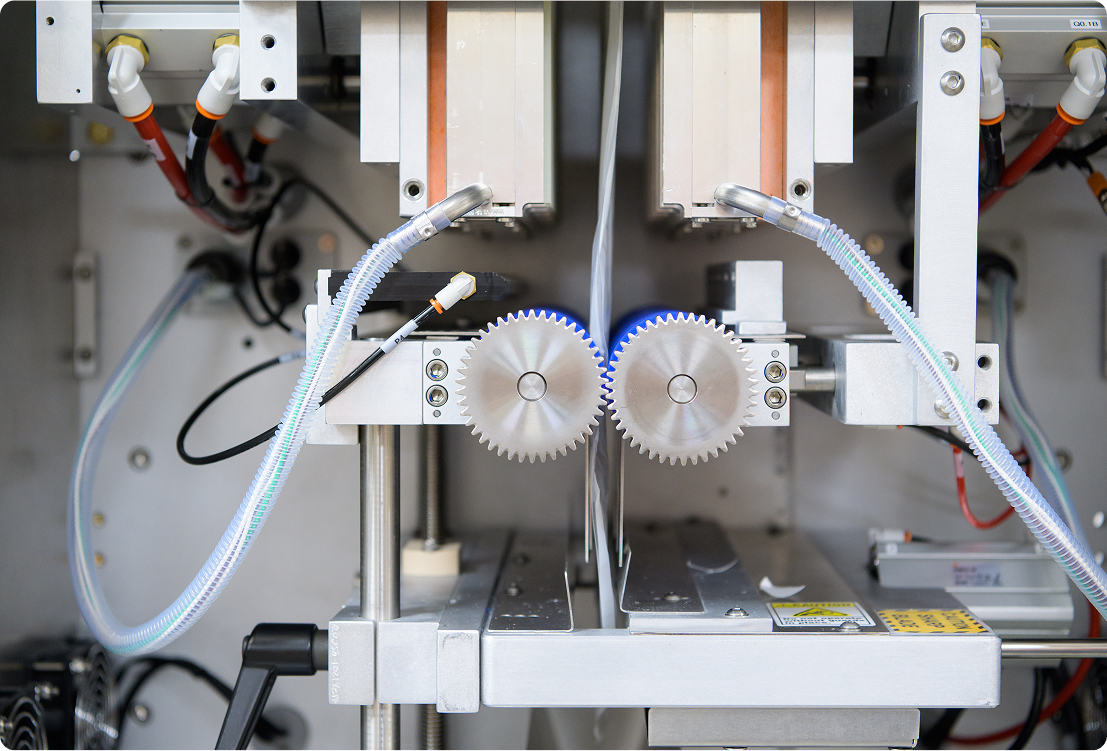
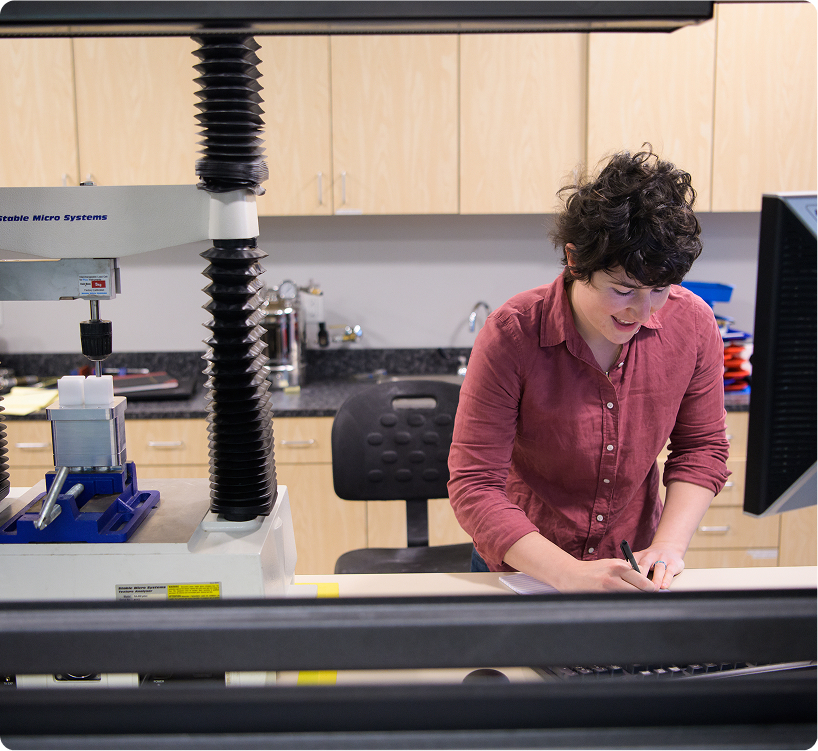
We turn early concepts into functional systems ready for testing, validation, and
transfer into manufacturing. Our team is equipped to manage projects and
programs to meet regulatory expectations and prepare for scale-up.
Our HQ and Main Design Center
Our Durham HQ functions as our primary hub for device development and design transfer. The facility features dedicated wet lab areas, prototyping labs, an on-site human factors suite, and specialized electromechanical prototyping space.

The Durham site is in close proximity to manufacturing in Pittsboro and Greensboro, enabling seamless design transfer.

This site specializes in concept design, DFM, prototyping, DV&V testing, package engineering, human factors, clinical builds and regulatory services.

Located in Chicago's HardTech Innovation Center
Gilero’s design center in Chicago, IL has allowed for us to expand and maintain our presence in the Midwest. This design center has access to prototyping space, machining capabilities, test labs, and additional equipment to help you bring your medical device to market.

Backed by a local team of engineers, designers, and project managers, we offer comprehensive support to bring groundbreaking medical technologies to life.

This site specializes in concept design, DFM, prototyping, DV&V testing, package engineering, human factors, clinical builds and regulatory services.

Our Office in the Southern California Medtech Hub
Southern California is a hub of innovation across many industries, and medical technology is rapidly becoming one of its most dynamic sectors. Our Carlsbad office connects West Coast clients with over two decades of expertise in medical device design and development.

Backed by a local team of engineers, designers, and project managers, we offer comprehensive support to bring groundbreaking medical technologies to life.

This site specializes in concept design, DFM, prototyping, DV&V testing, package engineering, human factors, clinical builds and regulatory services.