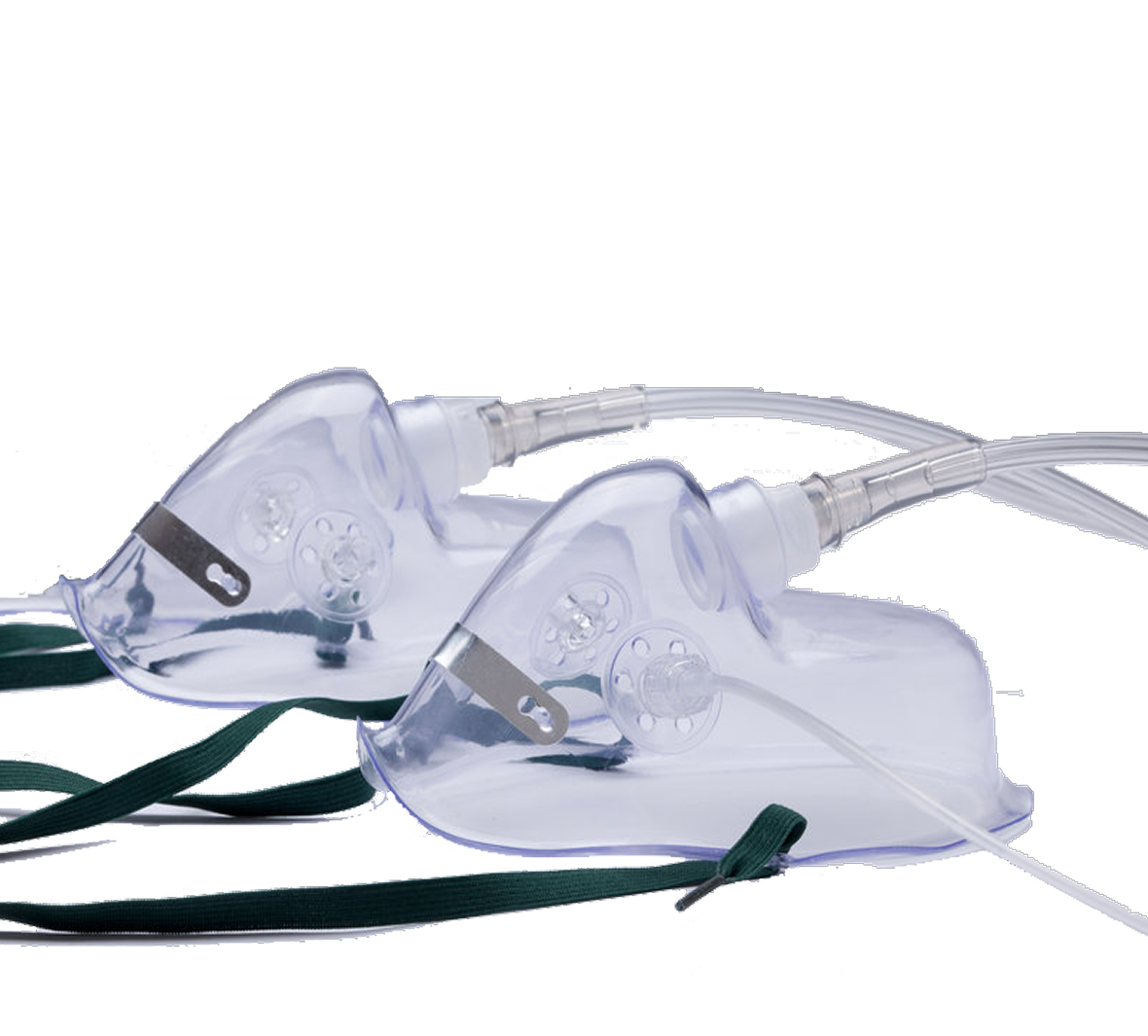
Whether you’re starting with a concept or need a reliable manufacturing partner, Gilero is built to deliver. From concept to product launch, we accelerate your path to market with integrated expertise in medical and pharmaceutical devices.
Our Expertise
Our Expertise in Action
Bringing a medical product to market is a complex process. Gilero streamlines development, compliance, and manufacturing across seven core areas, supporting a seamless and scalable path to commercialization.
Design & Development
Our design and development team brings deep experience across a wide range of medical devices, including drug delivery and diagnostic systems. We accelerate product development by leveraging our subject matter expertise to anticipate design risks early and streamline the transition from prototype to production.
Clinical & Small Batch Builds
We deliver tailored manufacturing services for companies developing products that require clinical trials and small batch builds. With flexible small-batch manufacturing and specialized regulatory support for drug-led combination products, we help bring new life-saving medical innovations into market.
Global Manufacturing Network
Explore Gilero’s expanded capabilities and global infrastructure and specialized manufacturing for medical devices, diagnostics, and drug delivery systems. Injection molding and cleanroom assembly facilities in the U.S., Mexico, Europe, and Asia enable Gilero to produce medical devices as finished goods.
Why Gilero
We accelerate the path to
market for innovative devices.
Gilero offers comprehensive, end-to-end solutions for various medical device applications including medtech, drug delivery, and diagnostics. From early-stage concept design to full-scale commercialization, we support projects across the entire product development lifecycle.
Our Work
Our Device Expertise
Gilero delivers end-to-end solutions for a wide variety of device types supporting clients through every product development phase.
Medtech
We design end-to-end solutions for MedTech applications, with capabilities spanning the design, development, and manufacturing of medical devices, pharmaceutical devices, and combination products. Our services support the full product lifecycle, from concept development and iterative design to regulatory planning, manufacturing, and post-market support.
Drug Delivery
We develop drug delivery systems with specialized capabilities in sustained-release and site-specific delivery designed to improve treatment performance and encourage adherence. Projects span a wide range of delivery methods, including pulmonary inhalers, ophthalmic devices, transdermal patches, reconstitution systems, and more.

Diagnostics
Gilero supports the development of diagnostic devices through tailored design, engineering, and manufacturing services. Capabilities include advanced engineering techniques such as microfluidics for precise sample handling and integrated connectivity for seamless data sharing.