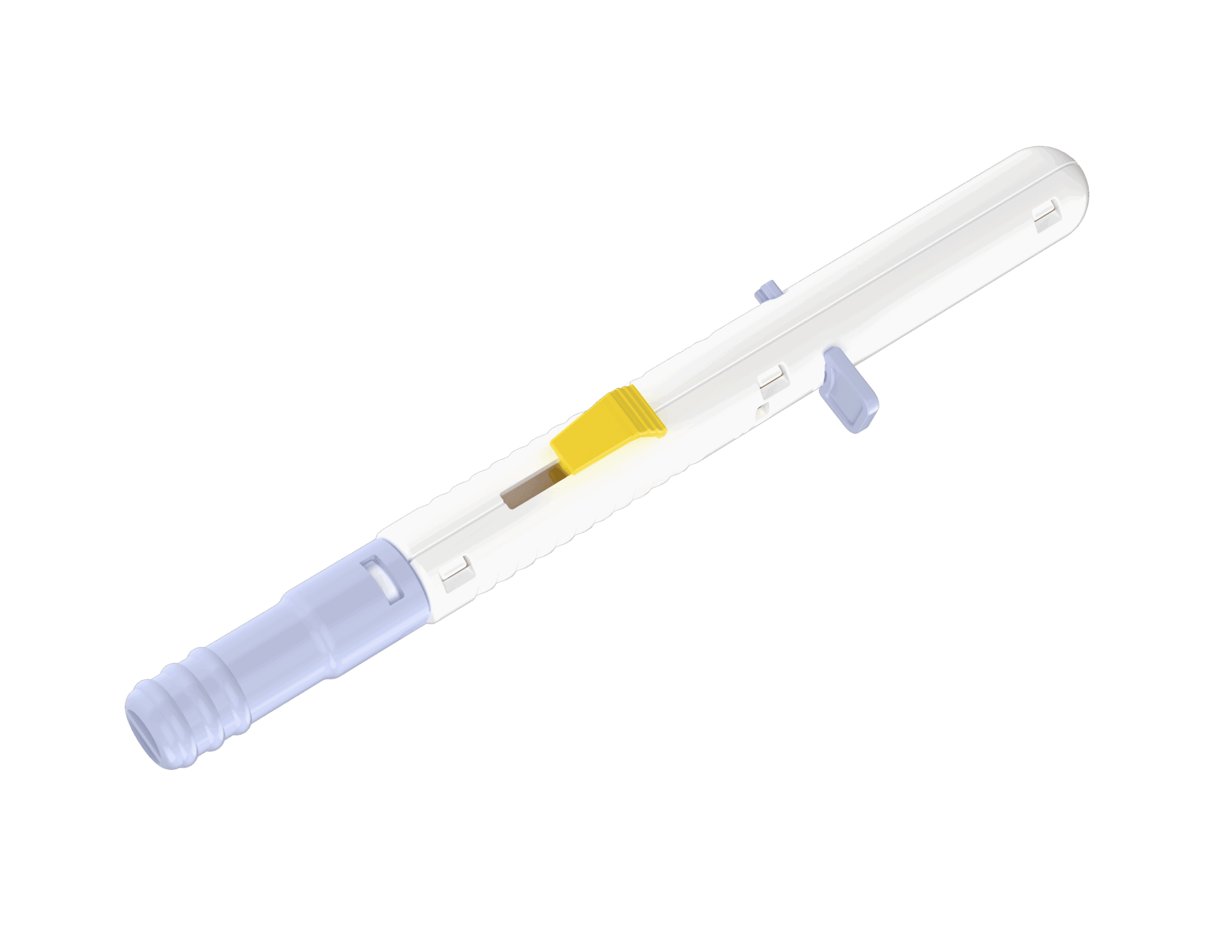
Gilero partnered with an ocular therapeutics company to develop a fully custom injection device capable of delivering an extended-release pharmaceutical implant into the vitreous for the treatment of wet age-related macular degeneration and glaucoma.
The Challenge
The client required a device that could reliably and safely deliver a novel pharmaceutical implant into the delicate ocular environment while meeting regulatory, human factors, and manufacturability requirements.
Our Approach
When the project began, Gilero dove in with concept development and rigorous proof-of-concept testing, shaping the vision for the new device. Once the initial ideas were validated, the team conducted a thorough IP Landscape Review and charted a clear pathway to bring the combination product to market. Drawing on their engineering expertise, Gilero designed a custom needle assembly, including the hub, needle, and cap, tailored to the client’s needs. To support early research, they then manufactured a small batch of over a thousand units, enabling critical toxicology testing and Human Factors KOL (Key Opinion Leaders) study.
The Result
A fully custom ocular injector, compatible with a novel pharmaceutical implant, now ready for further commercialization and integration into clinical use.